Demystifying Extractables and Leachables in the Pharmaceutical Industry: A Comprehensive Guide
July 26, 2025
In pharmaceutical product development, ensuring the safety, efficacy, and quality of the final product is of paramount importance. One critical but often overlooked area in this regard is the evaluation of extractables and leachables (E&L). These substances can originate from packaging materials, manufacturing components, or delivery systems and potentially contaminate drug products. Their presence, even in trace amounts, can lead to toxicity, loss of potency, or instability of the formulation.
The awareness around E&L studies has significantly grown, especially with the increasing use of complex drug delivery systems such as prefilled syringes, inhalers, and transdermal patches. Regulatory bodies like the FDA, EMA, and USP have mandated thorough E&L evaluations for certain classes of products, particularly parenterals and inhalation drugs.
This blog provides a detailed overview of extractables and leachables, highlighting their definitions, differences, importance, analytical evaluation techniques, the concept of Analytical Evaluation Threshold (AET), and the development of effective control strategies. It serves as a comprehensive resource for formulation scientists, regulatory professionals, and students aiming to understand and implement best practices in E&L studies.
What are Extractables and Leachables?
- Extractables
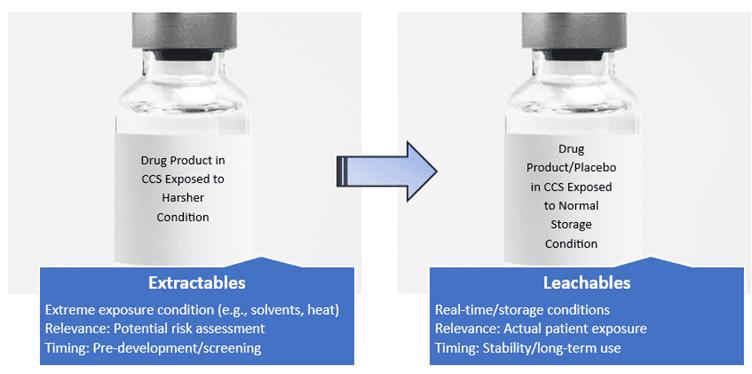
- Definition: chemical compounds that can be extracted from container closure systems or materials under aggressive (forced) conditions
- Typically evaluated using solvents under extreme conditions
- Purpose: to identify potential leachables
- Leachables
- Definition: chemical compounds that migrate into the drug product under normal storage/use conditions
- Can interact with the formulation and compromise safety, efficacy, or stability
What is Importance and Purpose of E&L Studies?
- Ensuring patient safety (toxicity, genotoxicity)
- Avoiding product recalls and regulatory non-compliance
- Supporting container closure system selection and qualification
- Required by regulators (FDA, EMA, USP, ICH, PQRI, ISO)
- Relevant to drug-device combinations (inhalers, prefilled syringes)
What are the Analytical Techniques for E&L Studies?
- Screening Techniques
- GC/GC-MS (Gas Chromatography-Mass Spectrometry): for volatile/semi-volatile organic compounds
- LC-MS (Liquid Chromatography-Mass Spectrometry): for non-volatile organics
- ICP-MS (Inductively Coupled Plasma-Mass Spectrometry): for elemental impurities (inorganics)
- FTIR (Fourier Transform Infrared Spectroscopy): for material characterization
- UV-Vis Spectroscopy: for aromatic or chromophoric compounds
- Quantification and Identification
- Analytical standards, internal standards, and calibration
- Use of high-resolution MS for unknowns
- Structural elucidation
- Extraction Methodologies
- Solvent selection: polar, non-polar, aqueous
- Extraction conditions: reflux, sonication, autoclaving
- Material selection: container closure, elastomers, process materials
- Leachable Study Design
- Time points and storage conditions
- Stability chamber monitoring
- Placebo or formulation matrix
- About Analytical Evaluation Threshold (AET):
- What is AET?
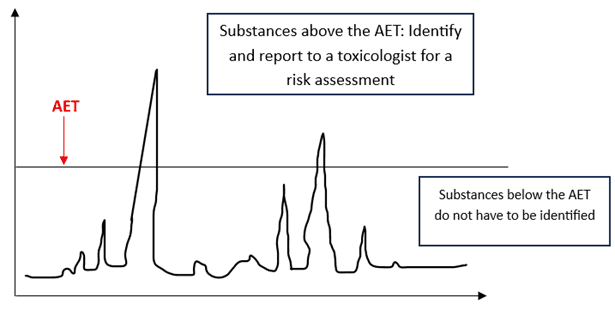
- Definition: the threshold above which analytical methods must identify and evaluate compounds. The AET is not a control threshold but a trigger point for identification and potential toxicological assessment.
- Definition: the threshold above which analytical methods must identify and evaluate compounds. The AET is not a control threshold but a trigger point for identification and potential toxicological assessment.
- Purpose of AET
- Determines sensitivity required for E&L studies
- Aligns study sensitivity with toxicological risk
- Purpose of AET
- Where:
- SCT (µg/day) is typically 1.5 µg/day for general thresholds (can vary)
- Dose: number of doses per day or container content

- What are the Risk Assessment Tools?
- Toxicological evaluation
- Use of PQRI Safety Thresholds (SCT, TTC)
- ICH M7-based impurity qualification
- What are Mitigation Measures?
- Material sourcing controls
- Manufacturing controls
- Packaging design changes
- USP Chapters: <661>, <1663>, <1664>
- PQRI Recommendations: for OINDPs and injectables
Regulatory Landscape and Guidelines: