Specific aspect of the CC Requests Related to Inactive Ingredients
Specific aspect of the CC Requests Related to Inactive Ingredients
July 12, 2025
What is Controlled Correspondence (CC):
Generic drug manufacturers and related industry can submit written inquiries to the Office of Generic Drugs (OGD) that are referred to as controlled correspondence. These are requests for information on a specific element of generic drug development or certain postapproval submission requirements. FDA and industry agreed to timelines for the review of controlled correspondence under the reauthorization of the Generic Drug User Fee Amendments (GDUFA III).
Specific aspect of the CC Requests Related to Inactive Ingredients:
- The Agency often receives requests for information pertaining to whether particular inactive ingredients present at higher levels than the maximums listed in the Agency’s Inactive Ingredient Database (IID) are permissible in a generic drug.
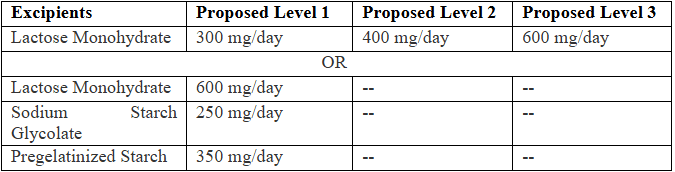
- A requestor can submit (1) a request that proposes three inactive ingredients with one level each, or (2) a request that proposes one inactive ingredient with three levels. Example given below.
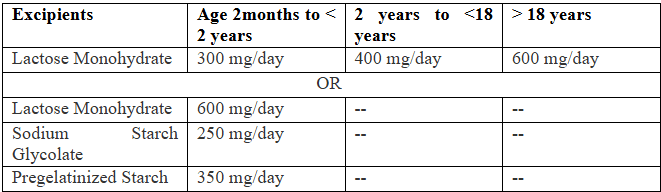
- If the drug product is indicated for the adult and pediatric populations, a requestor can submit (1) a request that proposes one inactive ingredient with one level for three different dosing ranges (based on body weight or age range specified in the RLD labeling), or (2) a request that proposes three inactive ingredients with one level for one dosage range. Example Given below.
- If the drug product is indicated for more than one route of administration, requests regarding inactive ingredients for each route of administration should be submitted in a separate controlled correspondence.
- If a requestor submits a range of levels for an inactive ingredient, the Agency only intends to review the highest proposed level in that range for that inactive ingredient. In addition, requestors should only submit the inactive ingredients they wish to be evaluated and their proposed levels and not the whole formulation.
- If levels of individual subcomponents (e.g., inactive ingredients that are composed of multiple subcomponents i.e., Flavours) are found within limits by the Agency when reviewed through a controlled correspondence, applicants should be aware that this does not necessarily mean the whole inactive ingredient will be found to be within acceptable limits during ANDA assessment.
- A requestor should wait for FDA’s response to the controlled correspondence before submitting a different request for consideration.
- IIG compliance of excipients rely on several factors like the dose, route of administration, duration of use, and patient population.
Resources:
- FDA’s Guidance document “Controlled Correspondence Related to Generic Drug Development”
- GDUFA III Commitment Letter
- Controlled Correspondence Coordinators at GenericDrugs@fda.hhs.gov.